Wavelength of light passing through the spectrometer, the intensity of
the light passing through the reference cell is measured. This is
usually referred to as
I0 - that's I for Intensity.

Fi
The intensity of the light passing through the sample cell is also measured for that wavelength - given the symbol,
I0 . If I is less than
I0 , then
the sample has absorbed some of the light
The absorbance of a transition depends on two external assumptions.
- The absorbance is directly proportional to the concentration (
c ) of the solution of the the sample used in the experiment. - The absorbance is directly proportional to the length of the light path (
l ), which is equal to the width of the cuvette.The Beer-Lambert Law
The absorbance (A ) is defined via the incident intensityI0 and transmitted intensityI by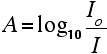
Absorption of light by a sample
A=log10(IoI)A=log10(IoI)A=log10(IoI)A=log10(IoI)
A = Log10 I0/I =elc
Where A is absorbance
e is the molar absorbtivity with units of L mol-1
cm-1
l is the path length of the sample - that is, the path length of the cuvette in which the sample is contained. We will express this measurement in centimeters.
c is the concentration of the compound in solution, expressed in mol L-1
l is the path length of the sample - that is, the path length of the cuvette in which the sample is contained. We will express this measurement in centimeters.
c is the concentration of the compound in solution, expressed in mol L-1
The proportion of the light absorbed will depend on how many molecules it interacts with. Suppose you have got a strongly colored organic dye. If it is in a reasonably concentrated solution, it will have a very high absorbance
Molar absorptivity
e = A/lc
Remember that the absorbance of a solution will vary as the concentration or the size of the container varies. Molar absorptivity compensates for this by dividing by both the concentration and the length of the solution that the light passes through. Essentially, it works out a value for what the absorbance would be under a standard set of conditions - the light traveling 1 cm through a solution of 1 mol dm-3.
That means that you can then make comparisons between one compound and another without having to worry about the concentration or solution length.
Values for molar absorptivity can vary hugely. For example, ethanol has two absorption peaks in its UV-visible spectrum - both in the ultra-violet. One of these corresponds to an electron being promoted from a lone pair on the oxygen into a pi anti-bonding orbital; the other from a pi bonding orbital into a pi anti-bonding orbital. because there are lots of molecules to interact with the light. However, in an incredibly dilute solution, it may be very difficult to see that it is colored at all. The absorbance is going to be very low.
Limitations / Deviations* :
It is capable of describing
absorption behavior of solutions containing relatively low amounts of
solutes dissolved in it (<10mM). When the concentration of the
analyte in the solution is high (>10mM), the analyte begins to behave
differently :
[i]Due to interactions with the solvent and other solute molecules.
[ii]Solute molecules can cause different charge distribution on their neighboring species.
[iii]Since UV-visible absorption is an electronic phenomenon, high concentrations would possibly result in a shift in the absorption wavelength of the analyte.
[iv] At times, even electrolyte concentrations (such as those present in buffers) play an important role in altering the charge distributions and affecting UV-visible absorbance.
[v] Some large ions or molecules show deviations even at very low concentrations. For example. methylene blue absorptivity at 436 nm fails to observe Beer Lambert law even at concentrations as low as 10μM.
[vi]High analyte concentrations MAY alter the refractive index (η) of the solution which in turn could affect the absorbance obtained. If addition of solute causes a significant change in the refractive index of the solution, then correct form of Beer Lambert is as:
A=εbc(ɳ^2+2)^2[i]Due to interactions with the solvent and other solute molecules.
[ii]Solute molecules can cause different charge distribution on their neighboring species.
[iii]Since UV-visible absorption is an electronic phenomenon, high concentrations would possibly result in a shift in the absorption wavelength of the analyte.
[iv] At times, even electrolyte concentrations (such as those present in buffers) play an important role in altering the charge distributions and affecting UV-visible absorbance.
[v] Some large ions or molecules show deviations even at very low concentrations. For example. methylene blue absorptivity at 436 nm fails to observe Beer Lambert law even at concentrations as low as 10μM.
[vi]High analyte concentrations MAY alter the refractive index (η) of the solution which in turn could affect the absorbance obtained. If addition of solute causes a significant change in the refractive index of the solution, then correct form of Beer Lambert is as:
This correction is normally not required below10mM concentrations .